Flame Test Sequence Photograph by Spl

Flame test Copper (II) chloride and potassium chloride YouTube
Flame test for copper salts. I have read from sources here, here and even in my textbook that Copper (specifically CuClX2 C u C l X 2 ) burns with a bluish green flame. All the sources mention that this is due to electron excitation and de-excitation in the orbitals of Cu. However, Why is copper the only transition element to give a coloured flame?
-Blue-real.jpg)
Amazing Flame Test
Copper, Cu 2+ Flame test colour: Blue-green: Question. A sample of an ionic compound close ionic compound An ionic compound occurs when a negative ion (an atom that has gained an electron).

Flame Test Sequence Photograph by Pixels
A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in the sample. Not all metal ions emit colour when heated in the gas burner. A flame test is the simplest way of identifying the presence of group 1 metal ions in the compound. For other metals, there are plenty of reliable techniques, but a flame.
CSI project Flame test
The metal salt's flame colour may be observed more easily when the yellow light is absorbed by the blue in the glass. Lithium - magenta red flame. Calcium - orange red flame. Potassium - lilac flame. Strontium - crimson red flame. Copper - blue or green flame (depends on the copper used) Sodium - yellow flame

Copper Flame Test Photograph by
Step 7. Unknown Crystals: Repeat the flame test steps using a small unknown crystal from the previous experiment as the sample. Use a clean, uncontaminated wire from the provided beaker. Record your observations in your notebook and use the results of the previous tests to determine which metal ions (if any) are present in the unknown crystals.

Copper Flame Test Photograph by Science Photo Library Pixels
The copper flame color is dependent on the presence of halide (I, F, Br, or Cl). The color can be used to detect halides by using copper oxide moistened with test solution. The outer darts of the flame are tinged with emerald-green. The selenium color is accompanied by the characteristic odor of rotting radishes.
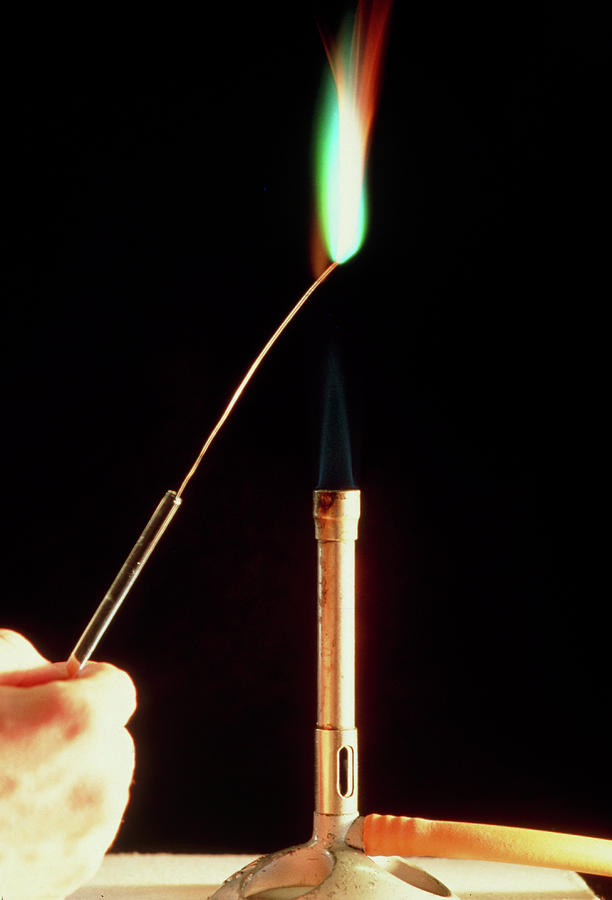
Copper Metal Flame Test Photograph by Andrew Mcclenaghan/science Photo Library. Fine Art America
The propane torch is a fire hazard, so a blue, flame resistant lab coat should be worn by the demonstrator. Keep flame pointed away from flammable material. SOP: N/A. Disposal (by Storeroom) All chemicals, except the copper chloride and strontium chloride, can be dissolved in water and flushed down the drain.

Copper Flame Test Stock Image C002/8023 Science Photo Library
All electromagnetic waves travel at the speed of light ( c ), or 2.998 × 108m / s. The relationship between the wavelength, frequency and speed of an electromagnetic wave is given by the equation: c = λ × ν. Electromagnetic radiation also occurs as discreet packets of energy (or quanta) called photons. The energy per photon (in Joules) is.

Copper flame test Stock Image C030/7629 Science Photo Library
The flame test is used to visually determine the identity of an unknown metal or metalloid ion based on the characteristic color the salt turns the flame of a Bunsen burner. The heat of the flame excites the electrons of the metals ions, causing them to emit visible light. Every element has a signature emission spectrum that can be used to differentiate between one element and another.

science chemistry flame test Fundamental Photographs The Art of Science
History The flame test carried out on a copper halide.The characteristic bluish-green color of the flame is due to the copper. Robert Bunsen invented the now-famous Bunsen burner in 1855, which was useful in flame tests due to its non-luminous flame that did not disrupt the colors emitted by the test materials. The Bunsen burner, combined with a prism (filtering the color interference of.

Flame Test Sequence Photograph by Science Photo Library Pixels
Calcium compounds result in an orange-red flame. Copper compounds result in a green flame. If a sample containing a mixture of ions is used some flame colours can be masked. Edexcel Chemistry. Topic 9 - Separate chemistry 2. Qualitative analysis: test for ions. 9.2Ca Describe flame tests to identify the following ions in solids: lithium ion, Li.

Copper Flame Test Photograph by David Taylor/science Photo Library Pixels
There are several ways of performing the flame test. Dissolve the sample in water or another solvent, soak a wooden splint in the liquid, and let it dry. Dip a Nichrome wire into a solid or liquid sample. Make a paste of a solid sample with hydrochloric acid (HCl) and dip a splint or wire into the paste. Dip a cotton swab into the sample.

Copper Sulfate Methanol Flame Test
Sodium Chloride: yellow flame; Strontium Chloride: red or crimson flame; Students should record the color on their activity sheets and use the visible light spectrum chart to estimate the wavelength or frequency of each. Put a strontium and a copper splint into the flame at the same time and ask students to identify which metals are present.

Flame Test Sequence Photograph by Spl
Flame Tests. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Not all metal ions give flame colors. For Group 1 compounds, flame tests are usually by far the easiest.

Flame test Copper (II) sulphate YouTube
Dr Thompson gives a 1 minute introduction to copper. Copper has a range of important applications. Copper is one of the few metals that can occur in nature i.

Copper metal flame test Stock Image A510/0022 Science Photo Library
FLAME TESTS. This page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Not all metal ions give flame colours. For Group 1 compounds, flame tests are usually by far the easiest way.